|
|
www.moyoway.com |
Buy your Certified Organic |
|
|
CLICK FOR NAKED www.moyoway.com |
Moyo is an African origin word which implies that the
"heart and mind act as one to cultivate the spirit”
Results of Two Year Study on Genetically Modified GM Corn and Pesticide
by Mark
(UK)

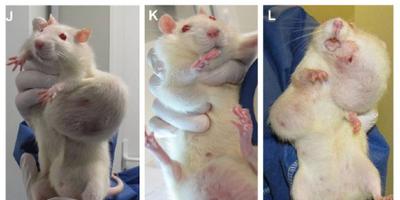
Are All of us Guinea Pigs (© Photo Jean-Baptiste Jaud /Graphisme LAGENCY)
This 2 year study, by Professor Gilles-Eric Séralini, professor of molecular biology at Caen university in France, was designed as a direct follow-up to another 90 day study concerning NK603 GMO Maize manufactured by Monsanto.
The initial study was done by Monsanto to support its application for regulatory authorisation to sell this GMO maize seed to the public. The Monsanto study was for a duration of 90 days based on a rat feeding trial and the results of this study were released in 2004.
In the same year, the European Food safety Authority (EFSA) allowed Monsanto to sell this seed into the European market. EFSA studied the results from Monsanto's test and found that the rats displayed "differences" to the ones not fed NK603 maize.
EFSA determined that the differences were “of no biological significance” and that the maize was as safe as non-GM maize.
Séralini’s team obtained Monsanto’s raw data from their 90 day test and re-analysed it. They found signs of liver and kidney toxicity in the GM-fed rats.
Séralini carried out his 2012 study on NK603 maize and Roundup (a herbicide manufactured by Monsanto) to see whether these initial findings of potential toxicity really were of no biological significance, as EFSA claimed, or whether they developed into more serious diseases.
Experiment Differences
The overall experimental design was similar to Monsanto’s, in order to make the two experiments comparable. Some of the differences were that Séralini’s experiment was longer (two years to Monsanto’s 90 days) and far more detailed in scope.
The detailed scope measured a larger number of health effects and was also designed to separate the effects of the GM maize from those of the herbicide it is engineered to tolerate, Roundup. What made this study different was that it was the first study on a GM crop to distinguish effects in this way.
The Difference Between a 90 Day Test and a 2 year Test
Over the 2 year period, Séralini found that both GM maize NK603 and Roundup caused serious kidney and liver damage and an increased and earlier development of tumours, leading to an increased rate of mortality.
Diseases like breast cancer in humans don't develop in 90 day tests and this comparison is important to highlight the fact that 90 day tests are simply too short when trying to determine the effects of GMO crops and their associated herbicides on humans and the environment.
Even in rats, serious diseases like organ damage and tumours take time to develop.
In addition to the long term effects on rats, another objective of Séralini’s study was to show that long-term chronic toxicity and carcinogenicity studies are needed on all GM foods before they are commercialised.
Presently, GM food crops are authorised on the basis of short (a maximum of 90-days) feeding studies, usually carried out in rats. Long-term studies are not required by regulators anywhere in the world.
But in Séralini’s study the first large tumours were only seen four months into the trial in the case of males and seven months in the case of females. Most other tumours were only detected after 18 months.
This shows that the 90-day tests routinely done on GM crops are not long enough to detect serious health effects that take time to develop, such as cancer and organ damage.
Conclusion of the 2 Year Study
As a comparison, a 90 day food test in a rat is equivalent to only 7 – 9 years in human terms. However, it needs to be remembered that humans can eat GM foods and be exposed to herbicide residues all their lives ... especially in view of the fact that GMO supporters advocate this product as a solution to feed our ever growing world.
EFSA's Response
In November 2012, EFSA released a statement on their website saying that Séralini’s study did not meet acceptable scientific standards.
They said that "Serious defects in the design and methodology of a paper by Séralini et al. mean it does not meet acceptable scientific standards and there is no need to re-examine previous safety evaluations of genetically modified maize NK603."
My Conclusion
A tumour is a tumour and if this GM maize and its herbicide has ANYTHING whatsoever to do with it, in anyway, over whatever period, in any amount, it needs to be removed from the world.
Cancer is increasing, especially in industrialised economies and nobody knows why.
Maybe the rats can tell us.
Comments for Results of Two Year Study on Genetically Modified GM Corn and Pesticide
|
||
|
||
|
||
|
||
|
||